- Home
- Publications
- PAGES Magazine
- Boron In CaCO3 As a Record of Past Seawater Carbonate Chemistry
Boron in CaCO3 as a record of past seawater carbonate chemistry
Henehan MJ & Jurikova H
Past Global Changes Magazine
27(2)
58-59
2019
Michael J. Henehan and Hana Jurikova
Boron incorporated in marine biogenic carbonates records the pH of seawater during precipitation. From reconstructing atmospheric CO2 beyond ice-core records to deciphering the ocean's role in storing and releasing carbon, boron is proving to be a vital tool in paleoclimate research.
Around a third of anthropogenic CO2 released to date has been taken up by the ocean. Its future capacity to sequester carbon, however, given potentially dynamic biogeochemical feedbacks, is unclear. Studies of the geological past provide numerous examples of how the ocean regulates and moderates atmospheric CO2 levels. To learn from these, however, we need effective recorders of the ocean's carbonate system. Boron (B)-based proxies – namely B/Ca ratios and the boron isotope (δ11B)-pH proxy applied to marine carbonate archives are among the most promising tools for reconstructing past ocean carbonate chemistry and atmospheric CO2. Here we briefly summarize some of the progress, problems, and prospects in the field.
Chemical basis of boron (B) proxies
In short, B-based proxies rely on the predictable pH-dependent speciation of dissolved B in seawater, between borate ion (B(OH)4-, prevalent at higher pH) and boric acid (B(OH)3, prevalent at lower pH), as shown in Figure 1. The B/Ca proxy works on the assumption that the more of the charged borate ion there is in solution (due to higher pH and lower CO2), the more B will be incorporated into the skeletal CaCO3 of marine calcifiers. The δ11B-pH proxy instead leverages the constant isotope fractionation associated with borate ion and boric acid speciation. This fractionation results in a predictable relationship between the δ11B of borate (the species incorporated into biogenic CaCO3) and pH. This foundation in aqueous chemistry has contributed to the considerable success of B-based proxies to date.
The B/Ca proxy
The B/Ca proxy is attractive in that the analytical method is simpler, and it requires less sample material than the δ11B-pH proxy. However, the outlook for this proxy is, at present, mixed. In planktic foraminifera, a host of environmental controls are now known to influence how much of the borate present in solution at any given pH is ultimately incorporated into calcite. These include salinity, ambient phosphorous concentration, light levels, and calcification rate (e.g. Allen and Hönisch 2012; Babila et al. 2014; Henehan et al. 2015; Salmon et al. 2016). Clearly, this complicates the use of B/Ca in planktic foraminifera as a straightforward pH proxy. Indeed, high-profile early applications of the proxy to reconstruct surface-ocean pH and hence atmospheric CO2 (Tripati et al. 2009) have since been shown to have been driven by secondary parameters involved in calculation, rather than the measured B/Ca data itself (Allen and Hönisch 2012). On the other hand, in deep-sea benthic foraminifera strong empirical relationships are observed between B/Ca and bottom-water carbonate saturation (∆[CO32-]; Yu and Elderfield 2007). This has been valuable in tracking the migration of CO2-rich deep-water bodies, and for the most part these reconstructions have been consistent with independent observations (e.g. 14C, δ13C, deep-sea coral δ11B). Collinearity between salinity, phosphorous, and ∆[CO32-] within benthic foraminiferal B/Ca calibration datasets (Henehan 2013), however, means some of the non-carbonate system controls seen in planktic foraminifera could still play a role.
On a more positive note, for all of its documented competing controls, in many geological records B/Ca does appear to behave like a pH proxy. For instance, at the Paleocene-Eocene Thermal Maximum, B/Ca declines in tandem with excursions in δ11B (Penman et al. 2014), suggesting that in some settings planktic foraminiferal B/Ca ratios can be at least qualitatively informative. It is thus premature at this point to discount the proxy entirely.
The boron isotope-pH proxy
Using boron isotopes circumvents many issues associated with B/Ca, allowing for quantitative reconstruction of pH and CO2. For example, diagenetic recrystallisation of fossil CaCO3 may result in loss of B (thus changing B/Ca), but the isotopic composition of the remaining B is unaffected (Edgar et al. 2015). Furthermore, factors like temperature and salinity have no competing effects on δ11B outside of their well-understood quantifiable effect on aqueous B speciation (e.g. Henehan et al. 2016). Most importantly, the dominant control of seawater pH on δ11B has been repeatedly demonstrated. For example, pH reconstructed from the δ11B of core-top deep-sea benthic foraminifera closely matches the pH of the water in which they grew (Rae et al. 2011), indicating the sole incorporation of borate into foraminifera and supporting the chemical foundation of the proxy.
For other calcifiers, although the control of pH on δ11B is clear, skeletal carbonate rarely records the δ11B of ambient seawater borate (δ11Bborate) exactly. Instead, their δ11B reflects a combination of δ11Bborate and a superimposed (typically species-specific) physiologically induced offset, termed a "vital effect". In the case of corals, this vital effect reflects the pH to which the calcifying fluid has been raised, which in turn varies with bulk seawater pH (Venn et al. 2013). In brachiopods and bivalves the situation is perhaps more complex, but their δ11B demonstrably varies with ambient pH (e.g. Jurikova et al. 2019).
In planktic foraminifera – our primary archive of surface-water pH and atmospheric CO2 – vital effects are also ubiquitous (unlike in deep-sea benthic foraminifera). Although we know foraminifera also raise the pH of their internal calcifying fluid (Bentov et al. 2009), thus far the most compelling explanation for species-specific deviations from δ11Bborate is microenvironment alteration (e.g. Henehan et al. 2016). This framework recognizes that planktic foraminifera don’t "see" ambient seawater, but rather a layer of seawater immediately surrounding their shell that is too small for turbulent mixing. It predicts, and indeed explains why, symbiont-bearing foraminifera living in the euphotic zone record higher-than-ambient pH and δ11Bborate: because their photosynthetic symbionts take up CO2 from their microenvironment. Conversely, species living below the euphotic zone, or those that don't host symbionts, are surrounded by seawater that is richer in respired CO2, and hence lower in pH. This also explains the lack of vital effects in deep-sea benthic foraminifera, as their slow metabolic rates mean diffusion can keep pace with release of respired CO2.
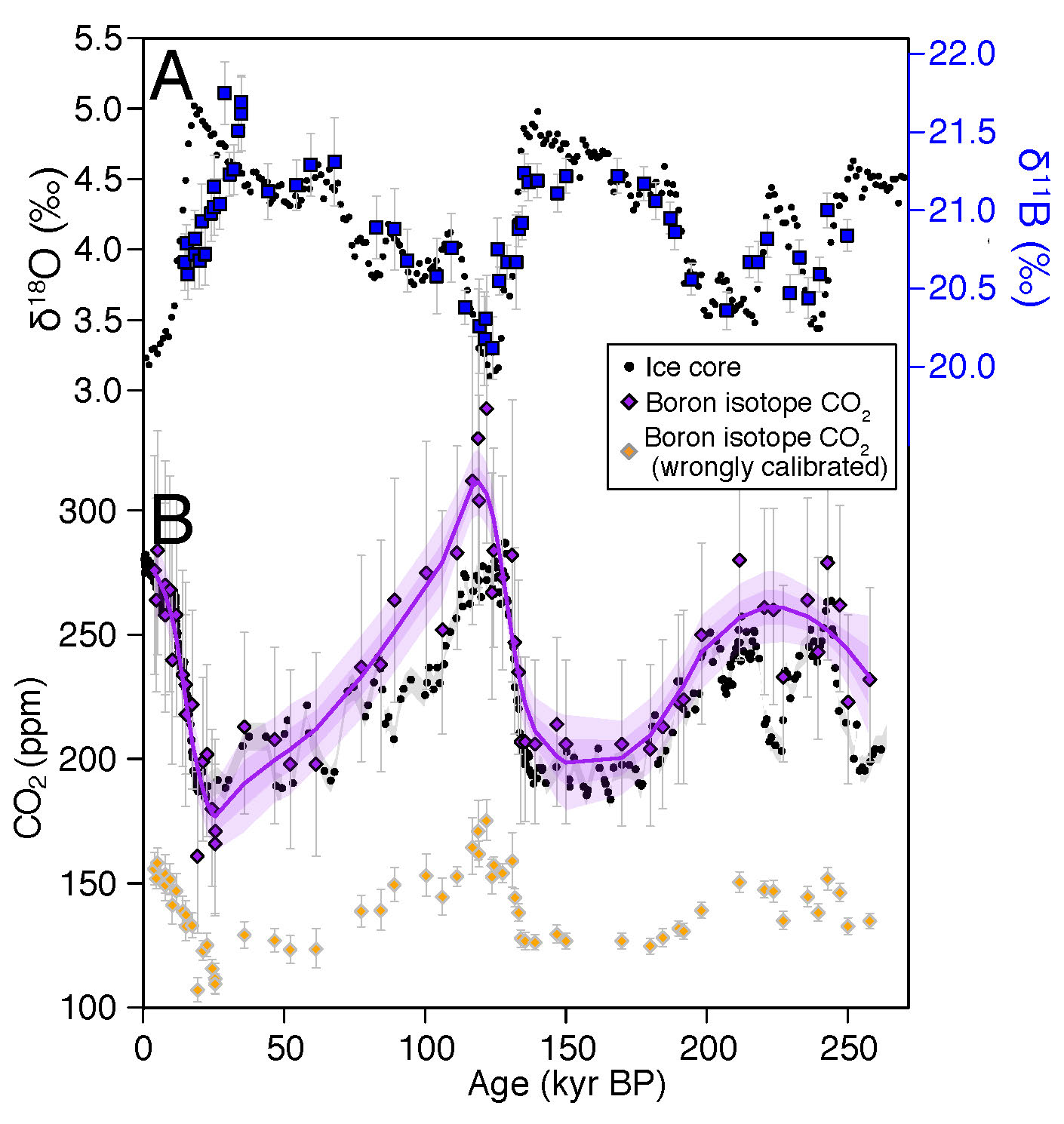
Although foraminiferal δ11B clearly varies with pH and CO2 regardless of vital effects (see e.g. data from Chalk et al. 2017 plotted in Fig. 2a), individual species differ significantly in their δ11B-pH (or more commonly δ11Bcalcite-δ11Bborate) calibrations. If a species' calibration is known, pH and CO2 values can be calculated from oligotrophic ocean regions with an accuracy and precision rivaled only by ice cores (Fig. 2b; in purple). However, without a calibration, for example with extinct species, quantifying absolute pH is more challenging. For example, if one erroneously applied a calibration derived for Orbulina universa to these same Globigerinoides ruber data from Chalk et al. (2017; Fig. 2c, in orange), reconstructed CO2 would be inaccurate. Thankfully, efforts to model and constrain vital effects in extinct species are ongoing (e.g. within the SWEET consortium; deepmip.org/sweet); these will reduce this source of uncertainty in deep-time reconstructions.
Beyond reconstructing atmospheric CO2 (by measuring δ11B in planktic foraminifera from regions where the atmosphere and surface ocean CO2 are in equilibrium), the δ11B-pH proxy can also be used to detect transient regional changes in air-sea CO2 disequilibrium. This has elucidated the role of changing ocean carbon storage in driving glacial-interglacial CO2 change, with CO2 release from the deep ocean to the atmosphere now known to have played a major role in pushing the Earth out of the last ice age (e.g. Martínez-Botí et al. 2015; Rae et al. 2018). There is considerable potential for such approaches to be applied in deeper time, for instance to investigate changes in ocean carbon storage during hyperthermal events. Ongoing analytical advances and shrinking sample size requirements mean these sorts of applications are coming into reach, potentially overhauling our understanding of how the ocean has influenced atmospheric CO2 through geological history.
affiliation
German Research Centre for Geosciences (GFZ), Helmholtz Centre Potsdam, Germany
contact
Michael Henehan: michael.henehan gfz-potsdam.de
gfz-potsdam.de
references
Allen KA, Hönisch B (2012) Earth Planet Sc Lett 345–348: 203–211
Babila TL et al. (2014) Earth Planet Sc Lett 404: 67–76
Bentov S et al. (2009) P Natl Acad Sci 106: 21500–21504
Chalk TB et al. (2017) P Natl Acad Sci 114: 13114–13119
Edgar KM et al. (2015) Geochim Cosmochim Acta 166: 189–209
Henehan MJ (2013) PhD thesis, University of Southampton, 348 pp
Henehan MJ et al. (2015) Geochem Geophys Geosystems 16: 1052–1069
Henehan MJ et al. (2016) Earth Planet Sc Lett 454: 282–292
Jurikova H et al. (2019) Geochim Cosmochim Acta 248: 370–386
Lisiecki LE, Raymo M (2005) Paleoceanography 20: PA1003
Lüthi D et al. (2008) Nature 453: 379-382
Martínez-Botí MA et al. (2015) Nature 518: 219–222
Penman DE et al. (2014) Paleoceanography 29: 357-369
Rae JWB et al. (2011) Earth Planet Sc Lett 302: 403–413
Rae JWB et al. (2018) Nature 562: 569–573
Salmon KH et al. (2016) Earth Planet Sc Lett 449: 372–381
Tripati AK et al. (2009) Science 326: 1394–1397