- Home
- Publications
- PAGES Magazine
- Ice-core Records of Atmospheric Composition and Chemistry
Ice-core records of atmospheric composition and chemistry
Banerjee A, Riddell-Young BE & Jongebloed UA
Past Global Changes Magazine
30(2)
104-105
2022
Asmita Banerjee![]() 1, Ben E. Riddell-Young
1, Ben E. Riddell-Young![]() 2 and Ursula A. Jongebloed
2 and Ursula A. Jongebloed![]() 3
3
All authors contributed equally and are considered joint first authors.
Ice cores record fundamental information about past atmospheric composition and chemistry, its intricate relationship with global climate, and recent changes to the atmosphere's composition due to anthropogenic activities.
As snow accumulates and compacts on ice sheets, ambient air is trapped within the ice, making glacial ice a direct archive of past atmospheric composition (McCrimmon et al. p. 112). The extraction and measurement of gases trapped in ice cores provide continuous, direct observations of atmospheric composition going back hundreds of thousands of years. These records show changes in atmospheric composition on timescales ranging from decades to hundreds of millennia (Martin et al. p. 100). Ice cores have provided high-resolution records of greenhouse gas (GHG) concentrations including carbon dioxide (CO2) and methane (CH4), and their influence on, and relation to, global climate.
In addition to trapped gases, ice cores also provide a unique archive of major ions and non-gaseous compounds including sulfate (SO42-, nitrate (NO3-), and halogens (e.g. chloride, bromide, iodide); atmospheric acidity; and other measurements that provide information about atmospheric chemistry and anthropogenic pollution. In the following sections, we describe these ice-core records of Earth's atmosphere.
Long-term records of greenhouse gases
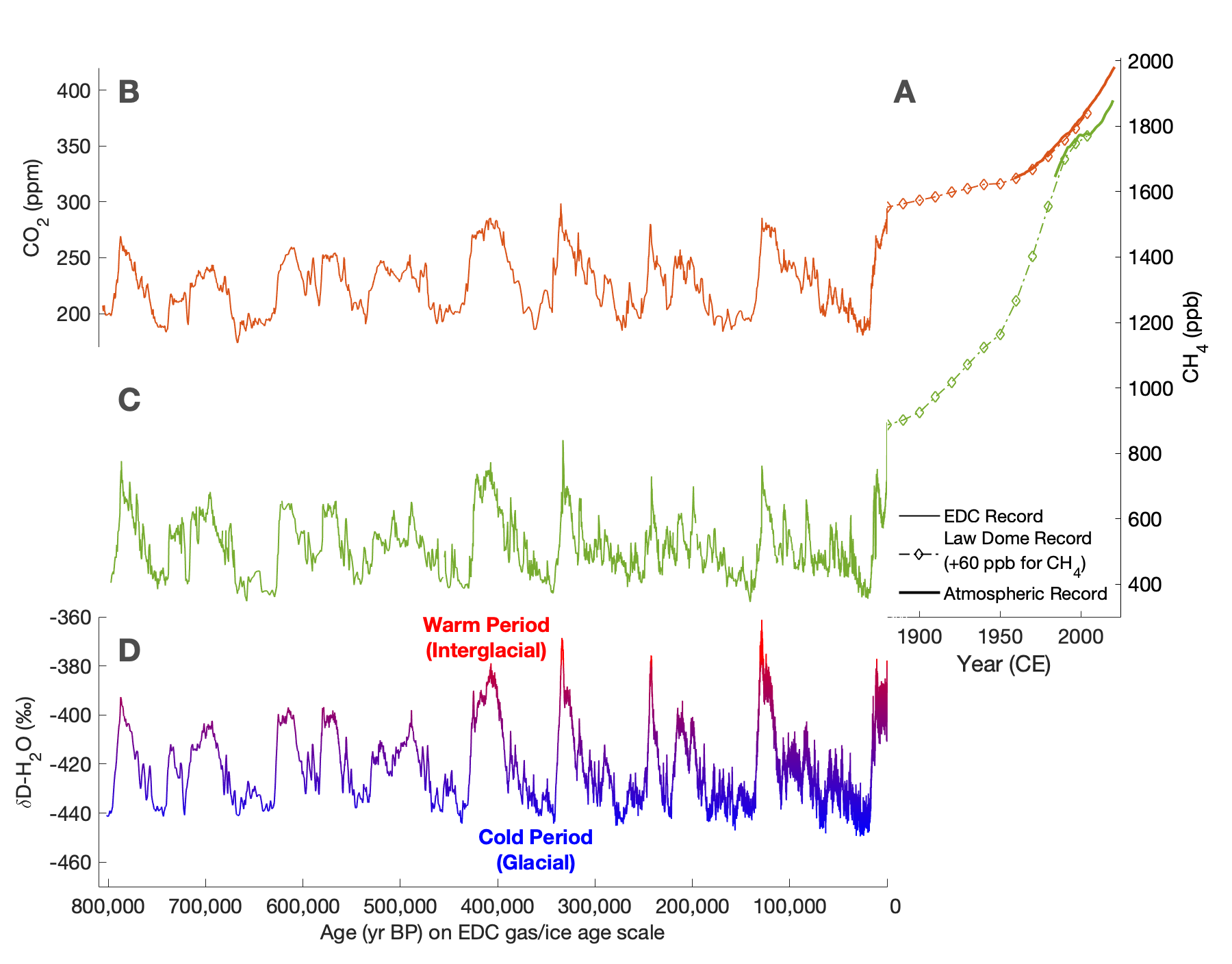
The fidelity of ice-core air as a record of past atmospheres is confirmed by the precise agreement between ice-core-derived records of GHGs and instrumental records over the last 40–60 years (Fig. 1a; Macfarling Meure et al. 2006). Ice cores from Antarctica have provided 800,000-year-long records of major GHGs in high resolution (CO2, CH4), covering eight complete glacial–interglacial cycles, with recent efforts aimed at recovering ice, and subsequently atmospheric composition, up to several million years old (Dahl-Jensen 2018). These data confirm that the modern atmospheric concentrations of GHGs and their rates of increase are unprecedented in at least the last 800,000 years.
Ice-core CO2 records have established the fundamental relationship between atmospheric CO2 and climate (derived from stable isotopes of H2O in the ice surrounding the bubbles, which are temperature proxies; Wendt et al. p. 102) on glacial–interglacial timescales (Fig. 1a, c; Jouzel et al. 2007). GHG records on centennial and millennial timescales provide strong evidence of abrupt changes to Earth's climate system and atmosphere. For example, ice-core CH4 records from Greenland and Antarctica reveal dramatic variability on decadal timescales that coincides with similarly abrupt Northern Hemisphere climate changes recorded in Greenland ice cores, highlighting the sensitivity of CH4 to abrupt climate change (Chappellaz et al. 1993).
Measurements of the stable and radioactive isotopic composition of atmospheric GHGs can reveal which sources contributed to changes in concentration over time. Because major GHG sources often have distinguishable isotopic compositions, variability in the strength of these sources over time had measurable impacts on the past atmospheric isotopic signature. Recently, the suite of trace gas measurements made on ice-core samples has expanded to include these isotope measurements, providing valuable constraints on the causes of past GHG variability and the complex dynamics of Earth's climate system. For example, records of stable isotopes in CO2 suggest that land-based CO2 sources caused abrupt CO2 rises during the last deglaciation (20,000-10,000 years ago), while ocean-based sources were responsible for a more gradual rise (Bauska et al. 2016). Records of CH4 isotopes strongly suggest that changes in microbial sources, rather than abrupt releases of geologic CH4, dominated the deglacial CH4 change (Dyonisius et al. 2020).
Modern records of anthropogenic change
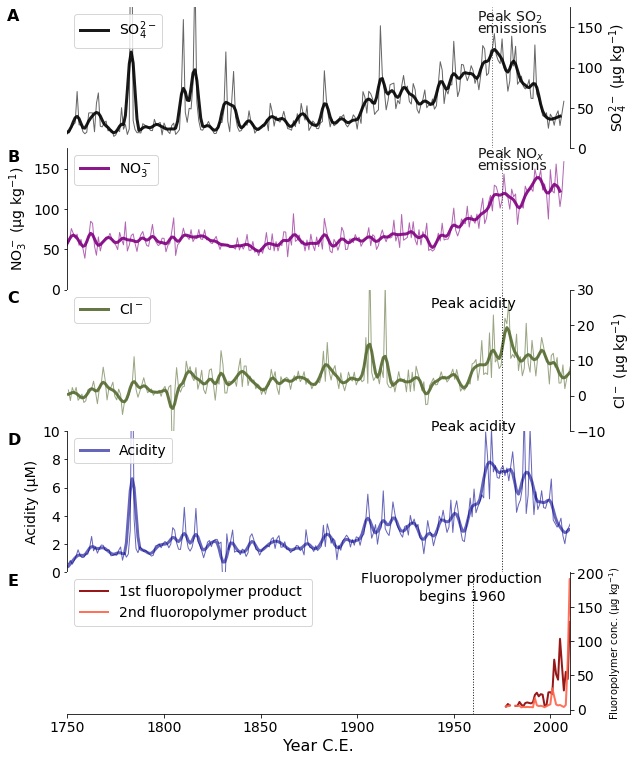
Ice cores preserve changes in atmospheric chemistry and pollution over human history (Wensman et al. p. 108). For example, they record how atmospheric sulfate, which causes acid rain and influences global climate, tripled between 1900 and 1980 due to fossil-fuel burning, and then declined from 1980 to present day following clean-air policies in North America and Europe (Mayewski et al. 1986; Fig. 2a). They also show how atmospheric nitrate concentrations have doubled since the 1950s due to increased NO and NO2 (collectively NOX) emissions from fossil-fuel combustion and agriculture, which have changed the biogeochemical cycling of nitrogen since the pre-industrial era (Hastings et al. 2009; Fig. 2b).
Ice cores also provide information about the past and current abundance of atmospheric oxidants, which are chemicals that react with air pollutants (e.g. SO2) and hydrocarbons (e.g. CH4), yielding products that can cool (e.g. SO4) or warm (e.g. CO2 the atmosphere. These reactions can determine the lifetime of GHGs such as CH4, so investigating how oxidants have changed can help estimate the warming potential of GHGs at different times in Earth's history. Although many oxidants such as ozone and the hydroxyl radical are too chemically reactive to be recorded in ice-core gas bubbles, proxies for these oxidants can indicate how oxidants have varied in the past. For example, clumped oxygen isotopes (i.e. 18O18O instead of 16O16O or 16O18O) constrain how ozone concentrations increased in the 20th century due to industrialization (Yeung et al. 2019).
Atmospheric halogens (elements including chlorine, bromine, and iodine) are some of the most reactive oxidants in the atmosphere and influence important species such as sulfate, volatile organic compounds, mercury, and ozone. It is difficult to know how atmospheric halogens have varied because measurements of reactive halogens have only been possible in the past few decades, but ice-core records combined with models can provide insight into how atmospheric halogen chemistry has changed due to anthropogenic pollution. For example, ice-core records of chlorine excess (i.e. chlorine that comes from a source other than sea salt; Fig. 2d) show how chlorine is correlated with atmospheric acidity (Fig. 2e) since the preindustrial, and atmospheric models indicate this correlation is due to acidity reacting with sea-salt aerosols (Zhai et al. 2021).
Ice cores also record pollutants that only exist due to anthropogenic activities. Figure 2e shows ice-core concentrations of perfluoroalkyl carboxylic acids, which are byproducts of refrigerants that have been found in ice cores starting in the mid-20th century and increasing rapidly after 1990 (Pickard et al. 2020). Records of these pollutants, along with concentrations of short-lived species such as sulfate, nitrate, and halogens, show how profoundly human activities have affected the chemistry and composition of the atmosphere, especially in the past 100 years.
Conclusions
Ice-core records provide unique archives of past changes in atmospheric composition and chemistry due to natural and anthropogenic causes. Ice-core gas records have provided information about past GHG concentrations and their relationship with global climate on glacial–interglacial and millennial timescales, as well as unprecedented increases in GHGs over the last century due to fossil-fuel burning. Analyses of major ions and other non-gaseous compounds have improved our understanding of anthropogenic pollution and its influence on atmospheric chemistry and climate. As older ice-core records are recovered and measurement techniques continue to improve, so too will our knowledge of past atmospheric composition and its interaction with climate and chemistry—knowledge that is essential for understanding the modern climate system and predicting future change.
affiliationS
1Department of Earth, Environmental and Planetary Sciences, Rice University, Houston, TX, USA
2College of Earth, Ocean, and Atmospheric Sciences, Oregon State University, Corvallis, USA
3Department of Atmospheric Sciences, University of Washington, Seattle, USA
contact
Asmita Banerjee: Asmita.Banerjee rice.edu
rice.edu
Ben Riddell-Young: riddellb oregonstate.edu
oregonstate.edu
Ursula Jongebloed: ujongebl uw.edu
uw.edu
references
Bauska TK et al. (2016) Proc Natl Acad Sci USA 113: 3465-3470
Chappellaz J et al. (1993) Nature 366: 443-445
Cole-Dai J et al. (2013) J Geophys Res Atmos 118: 7459-7466
Dahl-Jensen D (2018) Nat Geosci 11: 703-704
Dyonisius MN et al. (2020) Science 367: 907-910
Geng L et al. (2014) Proc Natl Acad Sci USA 111: 5808-5812
Hastings MG et al. (2009) Science 324: 1288
Hofman DJ et al. (2006) Tellus B 58: 614-619
Jouzel J et al. (2007) Science 317: 793-796
Macfarling Meure C et al. (2006) Geophys Res Lett 33: L14810
Mayewski PA et al. (1986) Science 232: 975-977
Pickard HM et al. (2020) Geophys Res Lett 47: e2020GL087535