- Home
- All News Overview
- Featured Article - Past Global Changes Magazine 31(2): A Whole Ocean Thermometer From Atmospheric Noble Gas Ratios
Monday, 19 February, 2024
By: Sarah Shackleton
Available in: Past Global Changes Magazine 31(2) "Young scientists at the leading edge of ice-core research"
> Access the contents of this issue
> Download this article as pdf
Marine-based reconstructions of ocean temperature have provided fundamental insight into past climate. The novel ice-core based proxy for mean ocean temperature from atmospheric noble gas ratios has demonstrated promise in furthering these insights.
Modern ocean warming
The oceans represent the largest source of thermal inertia in the climate system and play a key role in modulating the pacing of climate change. Measuring how much heat the oceans have taken up since the onset of the industrial era is important for understanding how the planet has responded to changes in our atmospheric composition driven by human activity. However, measuring total ocean warming is not an easy task, as heat uptake is spatially heterogeneous. Until recently, measurements of much of the ocean’s temperature have been sparse, and few measurements exist of ocean temperature below 2000 meters, where almost half of the ocean’s volume resides.
Probing ocean-temperature change before the instrumental era is an even greater challenge. Most of our information about past ocean temperature comes from marine-sediment records. However, as in the case of the instrumental era, ocean-temperature change at one location does not necessarily give you information about the global trend. In addition, most of our marine-sediment proxies for temperature provide information about sea-surface temperature, which represents a tiny fraction of the total ocean volume.
Changes in ocean temperature can also change its composition, including the quantity of gases dissolved in seawater. As the ocean warms, it can hold less gas, which leads to a net degassing of seawater as it surfaces and warms. This has important consequences for our atmosphere and climate; as seawater warms it can take up less CO2, leading to an increase in atmospheric CO2 and further warming.
Inert atmospheric gases trace ocean heat
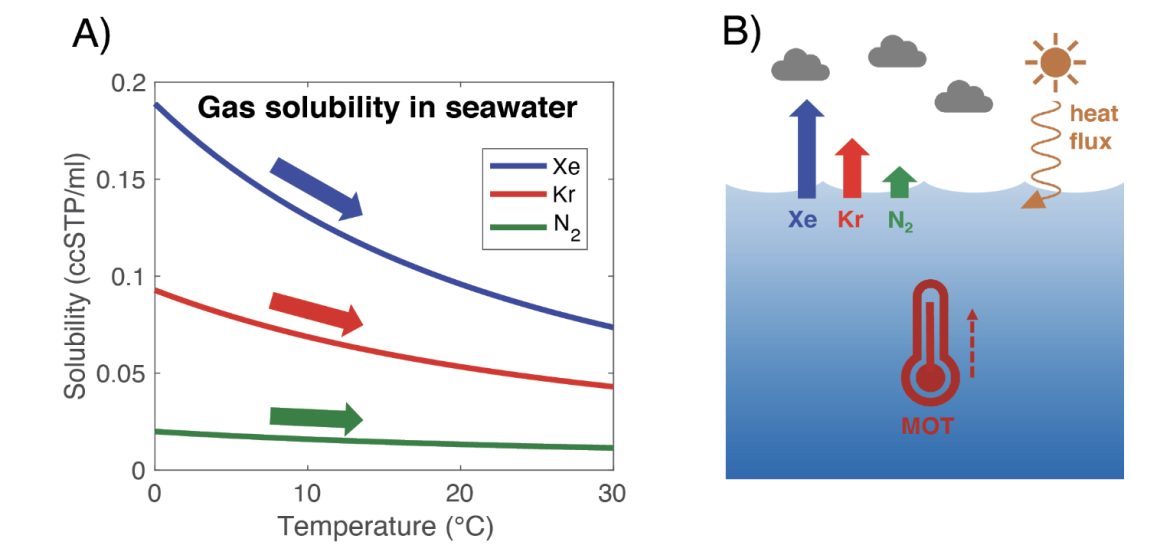
This temperature dependence of gas solubility in seawater also has consequences for the inert (or non-reactive) noble gases, including krypton (Kr) and xenon (Xe). The larger the noble gas, the higher its solubility in seawater, and the stronger the temperature dependence of that solubility (Fig. 1).
Between the ocean and atmosphere, about 5% of xenon is dissolved in the ocean and 95% resides in the atmosphere. At the average ocean temperature (3.5°C), the solubility of xenon changes by about 4% per °C of warming. Therefore, if the whole ocean warmed by 1°C, the concentration of xenon in the atmosphere would increase by roughly 0.2%. This may sound like a tiny change, especially given that xenon has an atmospheric concentration of only 87 parts per billion. However, these small changes may be measured.
In ice cores, we can measure the ratios of xenon and krypton in air bubbles with respect to one another (Xe/Kr) or relative to N2 (Xe/N2 and Kr/N2) to reconstruct global mean ocean temperature (Headly and Severinghaus 2007). While N2 is not entirely inert and may be converted to bio-available forms via nitrogen fixation, this bio-available nitrogen represents a miniscule portion (<0.01%) of the total nitrogen in the ocean and atmosphere; even a dramatic change to the nitrogen cycle would lead to a negligible change in the total N2 inventory. We may therefore treat N2 as an inert tracer, as we do Xe and Kr.
Why is this a whole ocean thermometer?
As implied above, seawater will only degas or ingas when it is at the surface and may equilibrate with the atmosphere, so it is not necessarily intuitive why the atmospheric noble gas ratios reflect mean ocean temperature change rather than that of the surface.
Figure 1: (A) Temperature dependence of inert gas solubilities in seawater (35 practical salinity units) and (B) schematic of the noble gas mean ocean temperature (MOT) proxy.